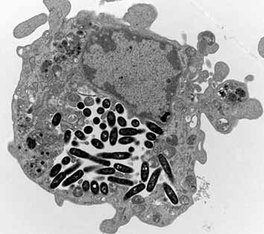
 Wild type Legionella in macrophage
Wild type Legionella in macrophage
Legionella pneumophila is a Gram negative pathogen that causes a severe pneumonia known as Legionnaires' disease. In the environment Legionella parasitizes protozoa. Humans become adventitiously infected through inhalation of Legionella containing aerosols. In the lungs, Legionella is taken up by alveolar macrophages (resident macrophages in the alveolar air spaces) where they replicate. Interestingly, the host immune response to infection is atypical for a bacterial infection. Instead of the usual neutrophil predominant response to acute infection, the air spaces fill up with an immune infiltrate consisting primarily of macrophages, the exact cell type that Legionella uses to replicate and propagate infection.
The cell biology of Legionella intracellular infection is fascinating and appears remarkably similar in both protozoa and mammalian macrophages. Legionella are taken up into a phagosome that fails to undergo normal endocytic maturation. Specifically, phagosomes never fuse with or mature into phagolysosomes. Rather they mature, in a type IV secretion system-dependent manner, into phagosomes with properties of rough endoplasmic reticulum. At this stage, the mature phagosomes become permissive for intracellular replication. Organisms multiply extensively until they fill up and then burst out of the host cell to continue the next round of replication.
The cell biology of Legionella intracellular infection is fascinating and appears remarkably similar in both protozoa and mammalian macrophages. Legionella are taken up into a phagosome that fails to undergo normal endocytic maturation. Specifically, phagosomes never fuse with or mature into phagolysosomes. Rather they mature, in a type IV secretion system-dependent manner, into phagosomes with properties of rough endoplasmic reticulum. At this stage, the mature phagosomes become permissive for intracellular replication. Organisms multiply extensively until they fill up and then burst out of the host cell to continue the next round of replication.
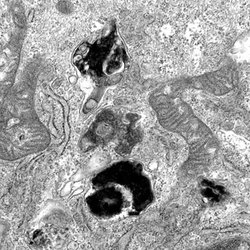 T4SS mutant destroyed
T4SS mutant destroyed
The type IV secretion system of Legionella pneumophila is complex, consisting of over 26 structural components, the so called Dot/Icm proteins. The system translocates "effectors" from the bacterial cytoplasm into the eukaryotic host cells. These effectors are directly responsible for altered phagosomal maturation. Type IV secretion system mutants do not replicate intracellularly. In contrast, knockout of a single translocated effector rarely demonstrates any phenotype. In explanation, it was eventually discovered that Legionella encodes a highly redundant set of effectors, potentially accommodating differences in environmental hosts, and inadvertently accounting for Legionella's ability to multiply intracellularly within organisms as diverse as Acanthamoeba castellani and humans. Amazingly, over 200 distinct Legionella translocated effectors have been identified, far more than in any other type IV secretion system-dependent pathogen. Interestingly, the type IV secretion system of Legionella is almost identical to that found in the human and mammalian pathogen, Coxiella burnetii. Yet, despite the similarity in T4SS components, translocated effectors and alterations in phagosomal biogenesis are not shared.